It is necessary to know the metal composition before welding in order to produce a successful weld.
Welders and metal workers must be able to identify various metal products so that proper work methods may be applied.
Any engineering drawing should be examined in order to determine the metal to be used and its heat treatment if required.
After some practice, the welder will learn that certain parts of machines or equipment are always cast iron, other parts are usually forgings, and so on.
Summary of Metal Composition and Identification Tests
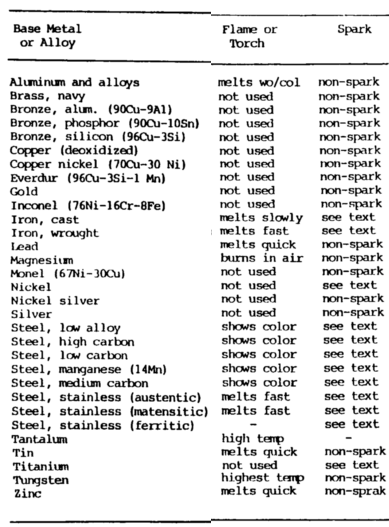
Metal Composition Tests
There are seven tests that can be performed in the shop to identify metals.
Six of the different tests are summarized in the table.
These should be supplemented by tables 7-1 and 7-2, which present physical and mechanical properties of metal, and table 7-3, which presents hardness data.
These tests are as follows:
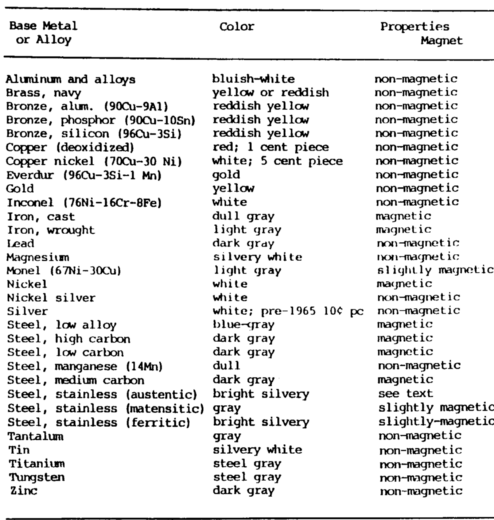
Appearance Test
The appearance metal composition test includes such things as color and appearance of machined as well as unmachined surfaces. Form and shape give definite clues as to the identity of the metal.
The shape can be descriptive; for example, shape includes such things as cast engine blocks, automobile bumpers, reinforcing rods, I beams or angle irons, pipes, and pipe fittings.
Form should be considered and may show how the part was rode, such as a casting with its obvious surface appearance and parting mold lines, or hot rolled wrought material, extruded or cold rolled with a smooth surface.
For example, pipe can be cast, in which case it would be cast iron, or wrought, which would normally be steel. Color provides a very strong clue in metal identification.
It can distinguish many metals, such as copper, brass, aluminum, magnesium, and precious metals. If metals are oxidized, the oxidation can be scraped off to determine the color of the unoxidized metal.
This helps to identify lead, magnesium, and even copper. The oxidation on steel, or rust, is usually a clue that can be used to separate plain carbon steel from the corrosion-resisting steel.
Fracture Test
Some metal can be quickly identified by looking at the surface of the broken part or by studying the chips produced with a hammer and chisel.
The surface will show the color of the base metal without oxidation. This will be true of copper, lead, and magnesium. In other cases, the coarseness or roughness of the broken surface is an indication of its structure.
The ease of breaking the part is also an indication of its ductility of lack of ductility. If the piece bends easily without breaking, it is one of the more ductile metals.
If it breaks easily with little or no bending, it is one of the brittle metals.
Spark Test
The spark metal composition test is a method of classifying steels and iron according to their composition by observing the sparks formed when the metal is held against a high speed grinding wheel.
This test does not replace chemical analysis but is a very convenient and fast method of sorting mixed steels whose spark characteristics are known. When held lightly against a grinding wheel, the different kinds of iron and steel produce sparks that vary in length, shape, and color.
The grinding wheel should be run to give a surface speed of at least 5000 ft (1525 m) per minute to get a good spark stream. Grinding wheels should be hard enough to wear for a reasonable length of time, yet soft enough to keep a free-cutting edge. Spark testing should be done in subdued light, since the color of the spark is important.
In all cases, it is best to use standard samples of metal for the purpose of comparing their sparks with that of the test sample.
Limitations
Spark metal composition testing is not of much use on nonferrous metals such as coppers, aluminums, and nickel-base alloys, since they do not exhibit spark streams of any significance. However, this is one way to separate ferrous and nonferrous metals.
Spark Test Results
Spark testing is not of much use on nonferrous metals such as coppers, aluminum, and nickel-base alloys, since they do not exhibit spark streams of any significance. However, this is one way to separate ferrous and nonferrous metals.
The spark resulting from the metal composition test should be directed downward and studied.
The color, shape, length, and activity of the sparks relate to the characteristics of the material being tested.
The spark stream has specific items which can be identified:
- The straight lines are called carrier lines.
- They are usually solid and continuous.
- At the end of the carrier line, they may divide into three short lines, or forks.
- If the spark stream divides into more lines at the end, it is called a sprig.
Sprigs also occur at different places along the carrier line. These are called either star or fan bursts. In some cases, the carrier line will enlarge slightly for a very short length, continue, and perhaps enlarge again for a short length.
When these heavier portions occur at the end of the carrier line, they are called spear points or buds. High sulfur creates these thicker spots in carrier lines and the spearheads.
Cast irons have extremely short streams, whereas low-carbon steels and most alloy steels have relatively long streams.
Steels usually have white to yellow color sparks, while cast irons are reddish to straw yellow.
A 0.15 percent carbon steel shows sparks in long streaks with some tendency to burst with a sparkler effect; a carbon tool steel exhibits pronounced bursting; and a steel with 1.00 percent carbon shows brilliant and minute explosions or sparklers. As the carbon content increases, the intensity of bursting increases.
Summary of Spark Test – Tables 7-5, Pictures a-c below:
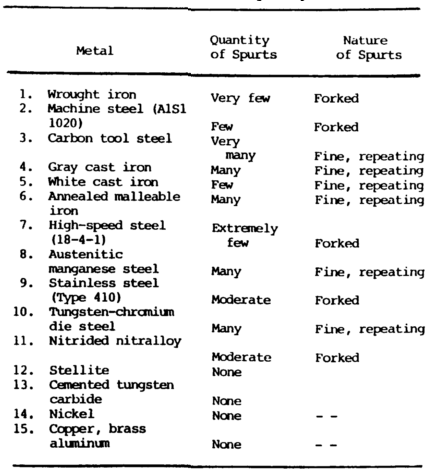
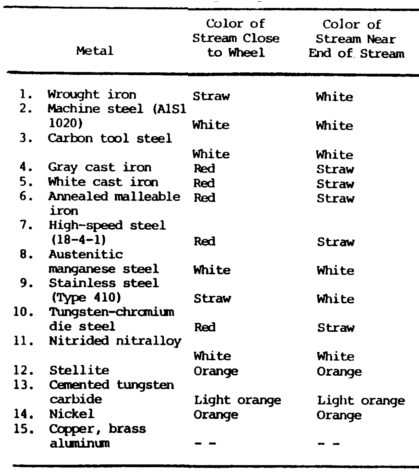
Advantages
One big advantage of this metal composition test is that it can be applied to metal in, all stages, bar stock in racks, machined forgings or finished parts.
The spark test is best conducted by holding the steel stationary and touching a high speed portable grinder to the specimen with sufficient pressure to throw a horizontal spark stream about 12.00 in. (30.48 cm) long and at right angles to the line of vision.
Wheel pressure against the work is important because increasing pressure will raise the temperature of the spark stream and give the appearance of higher carbon content. The sparks near and around the wheel, the middle of the spark stream, and the reaction of incandescent particles at the end of the spark stream should be observed.
Sparks produced by various metals are shown in figure 7-4.
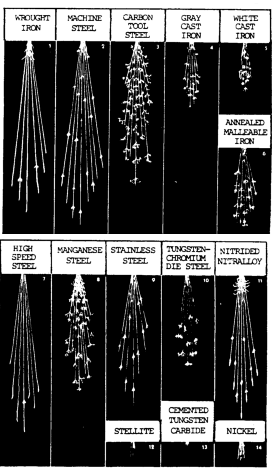
Caution: The torch metal composition test should be used with discretion, as it may damage teh part being tested. Additionally, magnesium may ignite when heated in the open atmosphere.
Torch Test
With the oxyacetylene torch, the welder can identify various metals by studying how fast the metal melts and how the puddle of molten metal and slag looks, as well as color changes during heating.
When a sharp corner of a white metal part is heated, the rate of melting can be an indication of its identity.
- If the material is aluminum: it will not melt until sufficient heat has been used because its high conductivity.
- If the part is zinc: the sharp corner will melt quickly, since zinc is not a good conductor.
- In the case of copper: if the sharp comer melts, it is normally de-oxidized copper.
If it does not melt until much heat has been applied, it is electrolytic copper.
Copper alloys, if composed of lead, will boil. - To distinguish aluminum from magnesium, apply the torch to filings.
- If magnesium: it will burn with a sparkling white flame. Steel will show characteristic colors before melting.
Magnetic Test
The magnetic metal composition test can be quickly performed using a small pocket magnet. With experience, it is possible to judge a strongly magnetic material from a slightly magnetic material. The nonmagnetic materials are easily recognized. Strongly magnetic materials include carbon and low-alloy steels, iron alloys, pure nickel, and martensitic stainless steels.
A slightly magnetic reaction is obtained from Monel and high-nickel alloys and the stainless steel of the 18 chrome 8 nickel type when cold worked, such as in a seamless tube.
Nonmagnetic materials include:
- Copper-base alloys
- Aluminum-base alloys
- Zinc-base alloys
- Annealed 18 chrome 8 nickel stainless
- Magnesium
- Precious metals
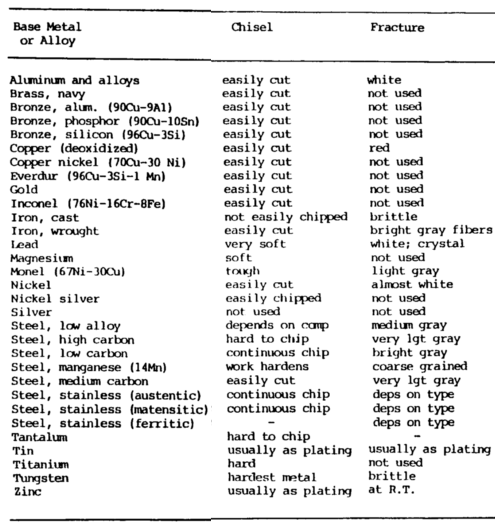
Chisel Test
The chip test or chisel metal composition test may also be used to identify metals. The only tools required are a banner and a cold chisel.
Use the cold chisel to hammer on the edge or corner of the material being examined. The ease of producing a chip is an indication of the hardness of the metal. If the chip is continuous, it is indicative of a ductile metal, whereas if chips break apart, it indicates a brittle material.
On such materials as aluminum, mild steel and malleable iron, the chips are continuous. They are easily chipped, and the chips do not tend to break apart.
The chips for gray cast iron are so brittle that they become small, broken fragments.
On high-carbon steel, the chips are hard to obtain because of the hardness of the material but can be continuous.
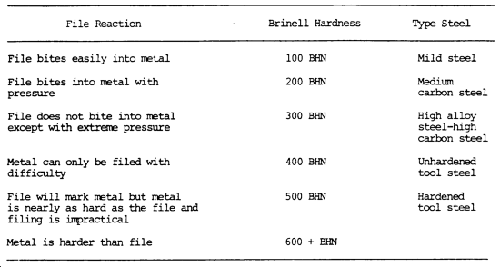
Hardness Test
Refer to table 7-3 for hardness values of the various metals, and to the above information on the three hardness tests that are commonly used.
A less precise hardness test is the file test. A summary of the reaction to filing, the approximate Brinell hardness, and the possible type of steel is shown in table 7-6. A sharp mill file must be used. It is assumed that the part is steel and the file test will help identify the type of steel.
Chemical Test
There are numerous chemical metal composition tests that can be made in the shop to identify some materials.
Monel can be distinguished from Inconel by one drop of nitric acid applied to the surface. It will turn blue-green on Monel but will show no reaction on Inconel. A few drops of a 45 percent phosphoric acid will bubble on low-chromium stainless steels.
Magnesium can be distinguished from aluminum using silver nitrate, which will leave a black deposit on magnesium, but not on aluminum. These tests can become complicated, and for this reason are not detailed further here.
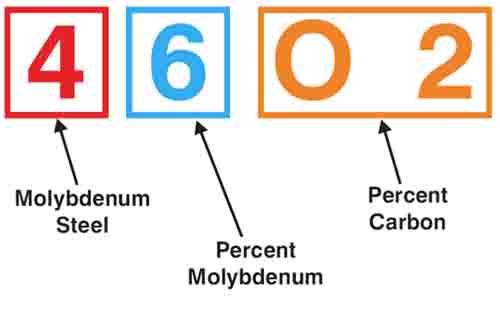
Color Code for Marking Steel Bars
SAE Metal Grading and Classifying System
The Bureau of Standards of the United States Department of Commerce has a color code for making steel bars.
The color markings provided in the code may be applied by painting the ends of bars. Solid colors usually mean carbon steel, while twin colors designate alloy and free-cutting steel.