Summary
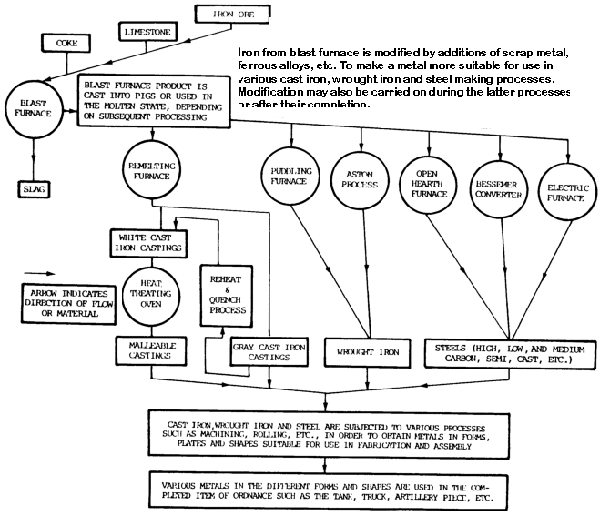
The basic substance used in ferrous metals such as steel and cast iron (gray and malleable) is iron.
It is used in the form of pig iron.
Iron is a base metal, meaning that it is an alloying agent in many different metals.
Iron is produced from iron ore that occurs chiefly in nature as an oxide, the two most important oxides being hematite and magnetite.
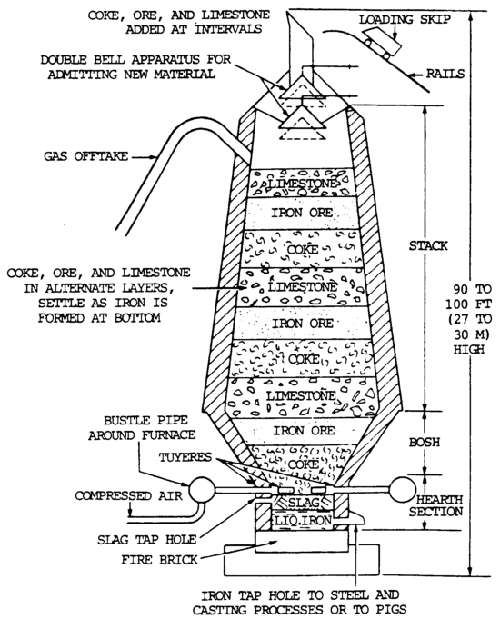
Iron ore is reduced to pig iron in a blast furnace, and the impurities are removed in the form of slag (figure 7-5).
Raw materials charged into the furnace include iron ore, coke, and limestone. The pig iron produced is used to manufacture steel or cast iron.
Common ferrous metals include:
- Stainless steels
- Tungsten carbide
- Carbon, tool and alloy steel
Ferrous Metal Composition, Properties and Characteristics
| Name | Composition | Properties & Characteristics |
|---|---|---|
| Cast Iron | Alloy of iron and 2.5% carbon, 1-3% silicone and traces of magnesium, sulfur & phosphorus | Hard skin, soft underneath but brittle. It corrodes by rusting |
| Mild Steel | Alloy of iron and 0.15-0.3% carbon | Tough, ductile & malleable. Good tensile strength but poor resistance to corrosion |
| Medium Carbon Steel | Alloy of iron and 0.35-0.7% carbon | Strong, hard & tough with high tensile strength but less ductile than mild steel |
| HIgh Carbon Steel | Alloy of iron and 0.7-1.5% carbon | Even harder than medium carbon steel and more brittle. Can be heat treated to make it harder& tougher |
| Stainless Steel | Alloy of iron and carbon with 16%-26% chromium, 8-22% nickel & 8% magnesium | Hard and tough, resists wear & corrosion |
| High-Speed Steel | Alloy of iron and 0.35-0.7% carbon (medium carbon steel) tungsten, chromium, vanadium & sometimes cobalt. | Very hard, high abrasion & heat resistance |
Steel
Plain carbon steel are ferrous metals that consist of iron and carbon.
Carbon is the hardening element. Tougher alloy steel contains other elements such as chromium, nickel, and molybdenum.
Cast iron is nothing more than basic carbon steel with more carbon added, along with silicon.
The carbon content range for steel is 0.03 to 1.7 percent, and 4.5 percent for cast iron.
Steel is produced in a variety of melting furnaces, such as open-hearth, Bessemer converter, crucible, electric-arc, and induction. Most carbon steel is made in open-hearth furnaces, while alloy steel is melted in electric-arc and induction furnaces.
Raw materials charged into the furnace include mixtures of iron ore, pig iron, limestone, and scrap. After melting has been completed, the steel is tapped from the furnace into a ladle and then poured into ingots or patterned molds.
The ingots are used to make large rectangular bars, which are reduced further by rolling operations. The molds are used for castings of any design.
Cast Iron

Cast iron is produced by melting a charge of pig iron, limestone, and coke in a cupola furnace. It is a brittle and hard metal with above-average levels of wear resistance. It is widely used in machine tools and automotive parts such as engines.
It is then poured into sand or alloy steel molds. When making gray cast iron castings, the molten metal in the mold is allowed to become solid and cool to room temperature in open air.
Malleable cast iron, on the other hand, is made from white cast iron, which is similar in content to gray cast iron except that malleable iron contains less carbon and silicon. White cast iron is annealed for more than 150 hours at temperatures ranging from 1500 to 1700°F (815 to 927°C).
The result is a product called malleable cast iron.
The desirable properties of cast iron are less than those of carbon steel because of the difference in chemical makeup and structure.
The carbon present in hardened steel is in solid solution, while cast iron contains free carbon, known as graphite.
In gray cast iron, the graphite is in flake form, while in malleable cast iron the graphite is in nodular (rounded) form.
This also accounts for the higher mechanical properties of malleable cast iron as compared with gray cast iron.
Iron Ore
Iron ore is smelted with coke and limestone in a blast furnace to remove the oxygen (the process of reduction) and earth foreign matter from it.
Limestone is used to combined with the earth matter to form a liquid slag. Coke is used to supply the carbon needed for the reduction and carburization of the ore. The iron ore, limestone, and coke are charged into the top of the furnace. Rapid combustion with a blast of preheated air into the smelter causes a chemical reaction, during which the oxygen is removed from the iron.
The iron melts, and the molten slag consisting of limestone flux and ash from the coke, together with compounds formed by the reaction of the flux with substances present in the ore, floats on the heavier iron liquid. Each material is then drawn off separately (figure 7-6).
All forms of cast iron, steel, and wrought iron consist of a mixture of iron, carbon, and other elements in small amounts.
Whether the metal is cast iron or steel depends entirely upon the amount of carbon in it.
The table below shows this principle:
| Item | Aprrox. % of Carbon | Condition of incorporated Carbon |
|---|---|---|
| Pig iron | 4% | Free & combined |
| White cast iron | 3.5% | Mostly combined |
| Gray cast iron | 2.5 – 4.5% | 0.6 to 0.9% free, 2.6 to 2.9% combined |
| Malleable cast iron | 2 – 3.5% | Free & combined |
| Tool steel | 0.9 – 1.7% | All combined |
| High-carbon steel | 0.5 – 0.9% | All combined |
| Medium-carbon steel | 0.3 – 0.5% | All combined |
| Cast steel | 0.15 – 0.6% | All combined |
| Low-carbon steel | up to 0.3% | All combined |
Cast iron differs from steel mainly because its excess of carbon (more than 1.7 percent) is distributed throughout as flakes of graphite, causing most of the remaining carbon to separate.
These particles of graphite form the paths through which failures occur, and are the reason why cast iron is brittle.
By carefully controlling the silicon content and the rate of cooling, it is possible to cause any definite amount of the carbon to separate as graphite or to remain combined. Thus, white, gray, and malleable cast iron are all produced from a similar base.
Wrought Iron

Wrought iron is one of the ferrous metals that is an alloy that is almost pure iron.
It is made from pig iron in a puddling furnace and has a carbon content of less than 0.08 percent. Carbon and other elements present in pig iron are taken out, leaving almost pure iron.
In the process of manufacture, some slag is mixed with iron to form a fibrous structure in which long stringers of slag, running lengthwise, are mixed with long threads of iron. Because of the presence of slag, wrought iron resists corrosion and oxidation, which cause rusting.
- Uses: Wrought iron is used for porch railings, fencing, farm implements, nails, barbed wire, chains, modern household furniture, ornaments and decorations.
- Capabilities: Wrought iron can be gas and arc welded, machined, plated, and is easily formed.
- Advantages: Wrought iron is bends easily when cold or when heated. It is easy to weld and rusts slowly.
- Limitations: Wrought iron has low hardness and low fatigue strength.
- Properties: Wrought iron has Brinell hardness number of 105; tensile strength of 35,000 psi; specific gravity of 7.7; melting point of 2750°F (1510°C); and is ductile and corrosion-resistant.
- Appearance test: The appearance of wrought iron is the same as that of rolled, low-carbon steel.
- Fracture test: Wrought iron has a fibrous structure due to threads of slag. As a result, it can be split in the direction in which the fibers run. The metal is soft and easily cut with a chisel, and is quite ductile. When nicked and bent, it acts like rolled steel. However, the break is very jagged due to its fibrous structure. Wrought iron cannot be hardened.
- Spark test: When wrought iron is ground, straw-colored sparks form near the grinding wheel, and change to white, forked sparklers near the end of the stream.
- Torch test: Wrought iron melts quietly without sparking. It has a peculiar slag coating with white lines that are oily or greasy in appearance.
Cast Iron (Gray, White and Malleable)
Cast iron is a man-made alloy of iron, carbon, and silicon. A portion of the carbon exists as free carbon or graphite. Total carbon content is between 1.7 and 4.5 percent.
- Uses: Cast iron is used for water pipes, machine tool castings, transmission housing, engine blocks, pistons, stove castings, etc.
- Capabilities: Cast iron may be brazed or bronze welded, gas and arc welded, hardened, or machined.
- Limitations: Cast iron must be preheated prior to welding. It cannot be worked cold.
- Properties:
- Cast iron has a Brinell hardness number of 150 to 220 (no alloys) and 300 to 600 (alloyed)
- Tensile strength of 25,000 to 50,000 psi (172,375 to 344,750 kPa) (no alloys) and 50,000 to 100,000 psi (344,750 to 689,500 kPa) (alloyed)
- Specific gravity of 7.6
- High compressive strength that is four times its tensile strength
- High rigidity
- Good wear resistance
- Fair corrosion resistance.
Other types of cast iron ferrous metals are described below:
Gray Cast Iron
If the molten pig iron is permitted to cool slowly, the chemical compound of iron and carbon breaks up to a certain extent. Much of the carbon separates as tiny flakes of graphite scattered throughout the metal.
This graphite-like carbon, as distinguished from combined carbon, causes the gray appearance of the fracture, which characterizes ordinary gray cast iron. Since graphite is an excellent lubricant, and the metal is shot throughout with tiny, flaky cleavages, gray cast iron is easy to machine but cannot withstand a heavy shock.
Gray cast iron consists of 90 to 94 percent metallic iron with a mixture of carbon, manganese, phosphorus, sulfur, and silicon.
Special high-strength grades of this metal also contain 0.75 to 1.50 percent nickel and 0.25 to 0.50 percent chromium or 0.25 to 1.25 percent molybdenum.
Commercial gray iron has 2.50 to 4.50 percent carbon. About 1 percent of the carbon is combined with the iron, while about 2.75 percent remains in the free or graphitic state.
In making gray cast iron, the silicon content is usually increased, since this allows the formation of graphitic carbon.
The combined carbon (iron carbide), which is a small percentage of the total carbon present in cast iron, is known as cementite.
In general, the more free carbon (graphitic carbon) present in cast iron, the lower the combined carbon content and the softer the iron.
Appearance Test
The unmachined surface of gray cast iron castings is a very dull gray in color and maybe somewhat roughened by the sand mold used in casting the part. Cast iron castings are rarely machined all over. Unmachined castings may be ground in places to remove rough edges.
Fracture Test
Nick a corner all around with a chisel or hacksaw and strike the corner with a sharp blow of the hammer.
The dark gray color of the broken surface is caused by fine black specks of carbon present in the form of graphite.
Cast iron breaks short when fractured. Small, brittle chips made with a chisel break off as soon as they are formed.
Spark Test
A small volume of dull-red sparks that follow a straight line close to the wheel are given off when this metal is spark tested.
These break up into many fine, repeated spurts that change to a straw color.
Torch Ferrous Metals Test
The torch test results in a puddle of molten metal that is quiet and has a jelly like consistency.
When the torch flame is raised, the depression in the surface of the molts-puddle disappears instantly.
A heavy, tough film forms on the surface as it melts. The molten puddle takes time to harden and gives off no sparks.
White Cast Iron
When gray cast iron is heated to the molten state, the carbon completely dissolves in the iron, probably combining chemically with it.
If this molten metal is cooled quickly, the two elements remain in the combined state, and white cast iron is formed. The carbon in this type of iron measures above 2.5 to 4.5 percent by weight, and is referred to as combined carbon.
White cast iron is very hard and brittle, often impossible to machine, and has a silvery-white fracture.
Malleable Cast Iron
Malleable cast iron is made by heating white cast iron from 1400 to 1700°F (760 and 927°C) for abut 150 hours in boxes containing hematite ore or iron scale.
This heating causes a part of the combined carbon to change into the free or uncombined state.
This free carbon separates in a different way from the carbon in gray cast iron and is called temper carbon.
It exists in the form of small, rounded particles of carbon which give malleable iron castings the ability to bend before breaking and to withstand shock better than gray cast iron.
The castings have properties more like those of pure iron: high strength, ductility, toughness, and ability to resist shock.
Malleable cast iron can be welded and brazed. Any welded part should be annealed after welding.
Appearance Test
The surface of malleable cast iron is very much like gray cast iron but is generally free from sand. It is dull gray and somewhat lighter in color than gray cast iron.
Fracture Test
When malleable cast iron is fractured, the central portion of the broken surface is dark gray with a bright, steel-like band at the edges.
The appearance of the fracture may best be described as a picture frame.
When of good quality, malleable cast iron is much tougher than other cast iron and does not break short when nicked.
Spark Test
When malleable cast iron is ground, the outer, bright layer gives off bright sparks like steel.
As the interior is reached, the sparks quickly change to a dull-red color near the wheel.
These sparks from the interior section are very much like those of cast iron; however, they are somewhat longer and are present in large volumes.
Torch Test
Molten malleable cast iron boils under the torch flame. After the flame has been withdrawn, the surface will be full of blowholes.
When fractured, the melted parts are very hard and brittle, having the appearance of white cast iron (they have been changed to white or chilled iron by melting and fairly rapid cooling).
The outside, bright, steel-like band gives off sparks, but the center does not.
Steel

A form of iron, steel is one of the ferrous metals that contain less carbon than cast iron, but considerably more than wrought iron. The carbon content is from 0.03 to 1.7 percent. Basic carbon steels are alloyed with other elements, such as chromium and nickel, to increase certain physical properties of the metal.
- Uses: Steel is used to make nails, rivets, gears, structural steel, roles, desks, hoods, fenders, chisels, hammers, etc.
- Capabilities: Steel can be machined, welded, and forged, all to varying degrees, depending on the type of steel.
- Limitations: Highly alloyed steel is difficult to produce.
- Properties: Steel has tensile strength of 45,000 psi (310,275 kPa) for low-carbon steel, 80,000 psi (551,600 kPa) for medium-carbon steel, 99,000 psi (692,605 kPa) for high-carbon steel, and 150,000 psi (1,034,250 kPa) for alloyed steel; and a melting point of 2800° F (1538°C).
- Low-carbon steel (carbon content up to 0.30 percent. This steel is soft and ductile, and can be rolled, punched, sheared, and worked when either hot or cold. It is easily machined and can readily be welded by all methods. It does not harden to any great amount; however, it can easily be case hardened.
Appearance Test
The appearance of the steel depends upon the method of preparation rather than upon composition. Cast steel has a relatively rough, dark-gray surface, except where it has been machined. Rolled steel has fine surface lines running in one direction. Forged steel is usually recognizable by its shape, hammer marks, or fins.
Fracture Test
When low-carbon steel is fractured, the color is bright crystalline gray. It is tough to chip or nick. Low carbon steel, wrought iron, and steel castings cannot be hardened.
Spark Test
The steel gives off sparks in long yellow-orange streaks, brighter than cast iron, that show some tendency to burst into white, forked sparklers.
Torch Test
The steel gives off sparks when melted, and hardens almost instantly.
Medium-carbon Steel (carbon content ranging from .30% to .50%)
This steel may be heat-treated after fabrication. It is used for general machining and forging of parts that require surface hardness and strength. It is made in bar form in the cold-rolled or the normalized and annealed condition. During welding, the weld zone will become hardened if cooled rapidly and must be stress-relieved after welding.
High-carbon Steel (carbon content ranging from .50% to .90%)
High-carbon steel (carbon content ranging from 0.50 to 0.90 percent).
This steel is used for the manufacture of drills, taps, dies, springs, and other machine tools and hand tools that are heat-treated after fabrication to develop the hard structure necessary to withstand high shear stress and wear.
It is manufactured in bar, sheet, and wire forms, and in the annealed or normalized condition in order to be suitable for machining before heat treatment.
This steel is difficult to weld because of the hardening effect of heat at the welded joint.
High-Carbon Steel Tests
Appearance Test
The unfinished surface of high-carbon steel is dark gray and similar to other steel. It is more expensive and is usually worked to produce a smooth surface finish.
Fracture test
High-carbon steel usually produces a very fine-grained fracture, whiter than low-carbon steel. Tool steel is harder and more brittle than plate steel or other low-carbon material.
High-carbon steel can be hardened by heating to a good red and quenching in water.
Spark test
High-carbon steel gives off a large volume of bright yellow-orange sparks.
Torch test
Molten high-carbon steel is brighter than low carbon steel, and the melting surface has a porous appearance. It sparks more freely than low-carbon (mild) steels, and the sparks are whiter.
High Carbon Tool Steel

Tool steel (carbon content ranging from 0.90 to 1.55 percent) is one of the ferrous metals that are used in the manufacture of chisels, shear blades, cutters, large taps, wood-turning tools, blacksmith’s tools, razors, and similar parts where high hardness is required to maintain a sharp cutting edge.
It is difficult to weld due to the high carbon content. A spark test shows a moderately large volume of white sparks having many fine, repeating bursts.
The advantages of tool steels are their ability to hold a cutting edge. Frequently used for applications such as drill bits punches, dies and cutters.
Cast Steel
Welding is difficult on steel castings containing over 0.30 percent carbon and 0.20 percent silicon.
Alloy steel castings containing nickel, molybdenum, or both of these metals, are easily welded if the carbon content is low.
Those containing chromium or vanadium are more difficult to weld.
Since manganese steel is nearly always used in the form of castings, it is also considered with cast steel. Its high resistance to wear is its most valuable property.
Cast Steel Tests
Appearance test
The surface of cast steel is brighter than cast or malleable iron and sometimes contains small, bubble-like depressions.
Fracture test
The color of a fracture in cast steel is bright crystalline gray. This steel is tough and does not break short. Steel castings are tougher than malleable iron, and chips made with a chisel curl up more. Manganese steel, however, is so tough that is cannot be cut with a chisel nor can it be machined.
Spark test
The sparks created from cast steel are much brighter than those from cast iron. Manganese steel gives off marks that explode, throwing off brilliant sparklers at right angles to the original path of the spark:
Torch test
When melted, cast steel sparks and hardens quickly.
Steel Forgings
Steel forgings may be of carbon or alloy steels. Alloy steel forgings are harder and more brittle than low carbon steels.
Steel Forging Tests
Appearance test
The surface of steel forgings is smooth. Where the surface of drop forgings has not been finished, there will be evidence of the fin that results from the metal squeezing out between the two forging dies.
This fin is removed by the trimming dies, but enough of the sheared surface remains for identification.
All forgings are covered with reddish-brown or black scale unless they have been purposely cleaned.
Fracture test
The color of a fracture in a steel forging varies from bright crystalline to silky gray. Chips are tough; and when a sample is nicked, it is harder to break than cast steel and has a finer grain.
Forgings may be of low-or high-carbon steel or of alloy steel. Tool steel is harder and more brittle than plate steel or other low-carbon material.
The fracture is usually whiter and finer-grained. Tool steel can be hardened by heating to a good red and then quenching in water.
Low-carbon steel, wrought iron, and steel castings cannot be usefully hardened.
Spark test
The sparks given off are long, yellow-orange streamers and are typical steel sparks. Sparks from high-carbon steel (machinery and tool steel) are much brighter than those from low-carbon steel.
Torch test
Steel forgings spark when melted, and the sparks increase in number and brightness as the carbon content becomes greater.
Alloy Steel

Alloy steel is one of the ferrous metals that is frequently recognizable by its use.
There are many varieties of alloy steel used in the manufacture of different types of equipment. They have greater strength and durability than carbon steel, and a given strength is secured with less material weight.
Manganese steel is a special alloy steel that is always used in the cast condition (see cast steel above).
Nickel, chromium, vanadium, tungsten, molybdenum, and silicon are the most common elements used in alloy steel.
- Chromium is used as an alloying element in carbon steels to increase hardenability, corrosion resistance, and shock resistance. It imparts high strength with little loss in ductility.
- Nickel increases the toughness, strength, and ductility of steels, and lowers the hardening temperatures so than an oil quench, rather than a water quench, is used for hardening.
- Manganese is used in steel to produce greater toughness, wear resistance, easier hot rolling, and forging. An increase in manganese content decreases the weldability of steel.
- Molybdenum increases hardenability, which is the depth of hardening possible through heat treatment. The impact fatigue property of the steel is improved with up to 0.60 percent molybdenum. Above 0.60 percent molybdenum, the impact fatigue property is impaired. Wear resistance is improved with molybdenum content above 0.75 percent. Molybdenum is sometimes combined with chromium, tungsten, or vanadium to obtain desired properties.
- Titanium and columbium (niobium) are used as additional alloying agents in low-carbon content, corrosion-resistant steels. They support resistance to intergranular corrosion after the metal is subjected to high temperatures for a prolonged time period.
- Tungsten, as an alloying element in tool steel, produces a fine, dense grain when used in small quantities. When used in larger quantities, from 17 to 20 percent, and in combination with other alloys, it produces steel that retains its hardness at high temperatures.
- Vanadium is used to help control grain size. It tends to increase hardenability and causes marked secondary hardness, yet resists tempering. It is also added to steel during manufacture to remove oxygen.
- Silicon is added to steel to obtain greater hardenability and corrosion resistance and is often used with manganese to obtain strong, tough steel. High-speed tool steels are usually special alloy compositions designed for cutting tools. The carbon content ranges from 0.70 to 0.80 percent. They are difficult to weld except by the furnace induction method.
- High yield strength, low alloy structural steels (often referred to as constructional alloy steels) are special low carbon steels containing specific small amounts of alloying elements. These steels are quenched and tempered to obtain a yield strength of 90,000 to 100,000 psi (620,550 to 689,500 kPa) and tensile strength of 100,000 to 140,000 psi (689,500 to 965,300 kPa), depending upon size and shape. Structural members fabricated of these high strength steels may have smaller cross-sectional areas than common structural steels and still have equal strength. In addition, these steels are more corrosion and abrasion-resistant. In a spark test, this alloy appears very similar to the low carbon steels.
NOTE: This type of steel is much tougher than low carbon steels, and shearing machines must have twice the capacity required for low carbon steels.
Appearance test
Alloy steel appears the same as drop-forged steel.
Fracture test
Alloy steel is usually very close-grained; at times the fracture appears velvety.
Spark test
Alloy steel produces characteristic sparks both in color and shape. Some of the more common alloys used in steel and their effects on the spark stream are as follows:
- Chromium. Steels containing 1 to 2 percent chromium have no outstanding features in the spark test. Chromium in large amounts shortens the spark stream length to one-half that of the same steel without chromium but does not appreciably affect the stream’s brightness. Other elements shorten the stream to the same extent and also make it duller. An 18 percent chromium, 8 percent nickel stainless steel produces a spark similar to that of wrought iron, but only half as long. Steel containing 14 percent chromium and no nickel produces a shorter version of the low-carbon spark. An 18 percent chromium, 2 percent carbon steel (chromium die steel) produces a spark similar to that of carbon tool steel, but one-third as long.
- Nickel. The nickel spark has a short, sharply defined dash of brilliant light just before the fork. In the amounts found in S. A. E. steels, nickel can be recognized only when the carbon content is so low that the bursts are not too noticeable.
- High chromium-nickel alloy (stainless) steels. The sparks given off during a spark test are straw colored near the grinding wheel and white near the end of the streak. There is a medium volume of streaks having a moderate number of forked bursts.
- Manganese. Steel containing this element produces a spark similar to a carbon steel spark. A moderate increase in manganese increases the volume of the spark stream and the force of the bursts. Steel containing more than the normal amount of manganese will spark in a manner similar to high-carbon steel with low manganese content.
- Molybdenum. Steel containing this element produces a characteristic spark with a detached arrowhead similar to that of wrought iron. It can be seen even in fairly strong carbon bursts. Molybdenum alloy steel contains nickel, chromium, or both.
- Molybdenum with other elements. When molybdenum and other elements are substituted for some of the tungsten in high-speed steel, the spark stream turns orange. Although other elements give off a red spark, there is enough difference in their color to tell them from a tungsten spark.
- Tungsten. Tungsten will impart a dull red color to the spark stream near the wheel. It also shortens the spark stream, decreases the size, or completely eliminates the carbon burst. Steel containing 10 percent tungsten causes short, curved, orange spear points at the end of the carrier lines. Still lower tungsten content causes small white bursts to appear at the end of the spear point. Carrier lines may be anything from dull red to orange in color, depending on the other elements present, if the tungsten content is not too high.
- Vanadium. Alloy steels containing vanadium produce sparks with a detached arrowhead at the end of the carrier line similar to those arising from molybdenum steels. The spark test is not positive for vanadium steels.
- High-speed tool steels. A spark test in these steels will impart a few long; forked sparks which are red near the wheel, and straw-colored near the end of the spark stream.
- Special steel. Plate steel is used in the manufacture of built-up welded structures such as gun carriages. In using nickel plate steel, it has been found that commercial grades of low-alloy structural steel of not over 0.25 percent carbon, and several containing no nickel at all, are better suited to welding than those with a maximum carbon content of 0.30 percent. Armor plate, a low carbon alloyed steel, is an example of this kind of plate. Such a plate is normally used in the “as rolled” condition. Electric arc welding with a covered electrode may require preheating of the metal, followed by proper stress-relieving heat treatment (post-heating), to produce a structure in which the welded joint has properties equal to those of the plate metal.